FL-301
(NBL-015)
Anti-CLDN18.2 

Anti-CLDN18.2 in gastric and pancreatic cancers
FL-301 antibody mechanism
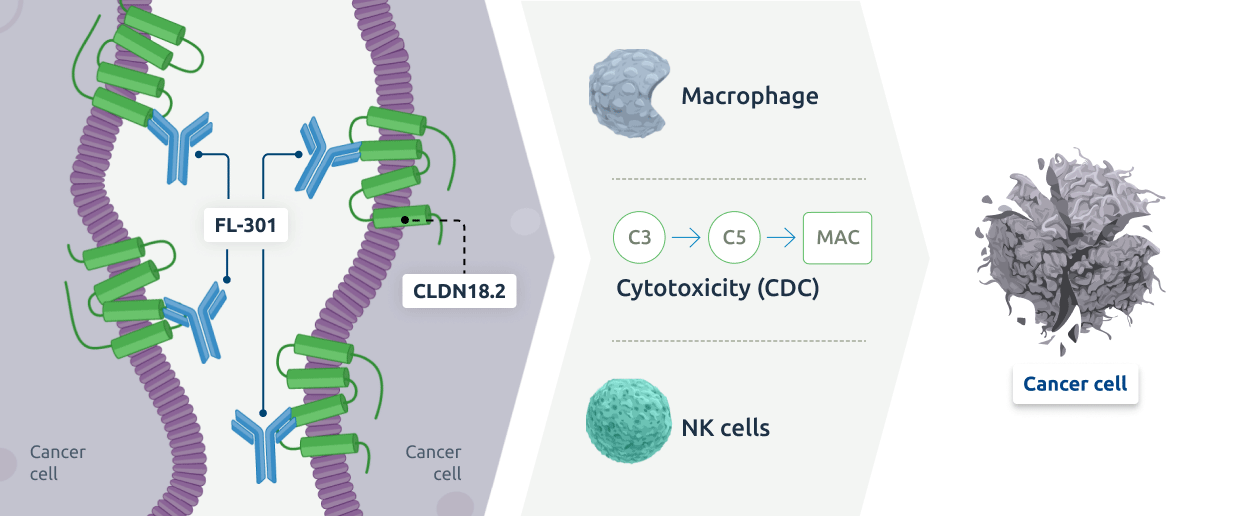
FL-301 is a fully human monoclonal antibody that binds to and blocks Claudin18.2. In nonclinical models, FL-301 was shown to have 10-20x higher affinity to Claudin18.2 than the benchmark antibody zolbetuximab and specificity to both gastric and pancreatic tumors.
Through Fc engineering, FL-301 has been designed with enhanced antibody dependent cellular cytotoxicity, complement dependent cytotoxicity, and antibody dependent cellular phagocytosis, which are three mechanisms that can lead to improved cancer cell killing and greater potency relative to zolbetuximab in nonclinical models.
Ongoing FL-301 (NBL-015) clinical trials
FL-301 (NBL-015) clinical trial
This clinical trial (NCT05153096) is a multicenter, open-label phase I clinical trial conducted in patients with CLDN18.2-positive advanced solid tumors, aiming to evaluate the safety, tolerability, pharmacokinetic characteristics and preliminary efficacy of FL-301 (NBL-015) in patient with advanced solid tumors.
This study consists of two stages: the stage I (dose escalation and dose expansion) and the stage II (NBL-015 monotherapy cohort expansion and combination of NBL-015 with standard treatment cohort expansion).

